
Calculate the concentration of nitric acid in moles per litre in a sample which has a density 1.41 g mL^-1 and the mass per cent of nitric acid in it being 69% .

16 Molarity calculations ideas | converting metric units, measurement conversions, model question paper

Molarity Dilution Problems Solution Stoichiometry Grams, Moles, Liters Volume Calculations Chemistry - YouTube

1 Example A 25.0 L sample was found to contain 26.7 g glucose. Express the concentration as ppm and mg/dL glucose. Solution A ppm is defined as g/mL, - ppt download

Calculating molarity units molar concentration of solutions practice questions on molarity how to make up a standard solution how to determine solubility gcse chemistry igcse KS4 science A level GCE AS A2




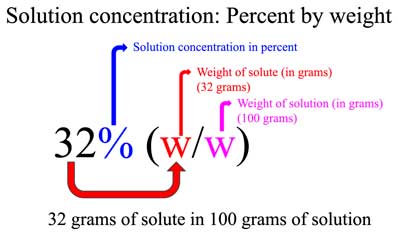



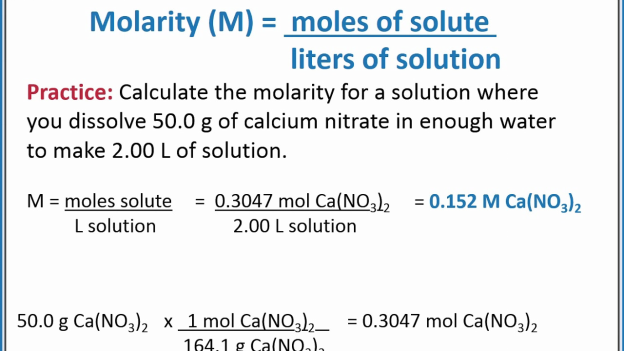



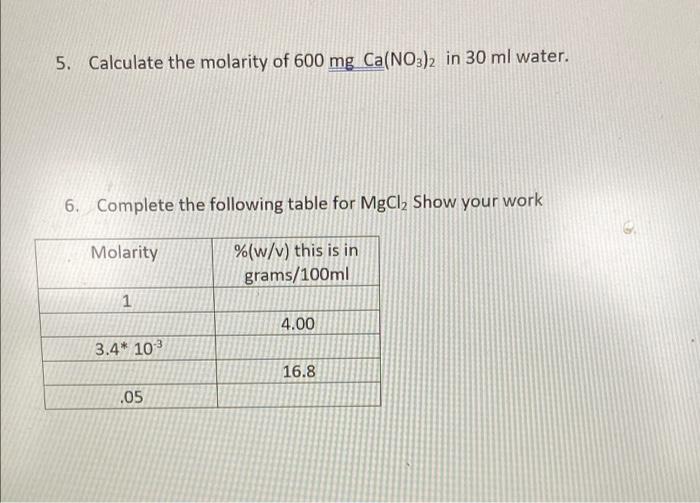
![Molarity Calculator [with Molar Formula] Molarity Calculator [with Molar Formula]](https://scrn-cdn.omnicalculator.com/chemistry/molarity@2.png)