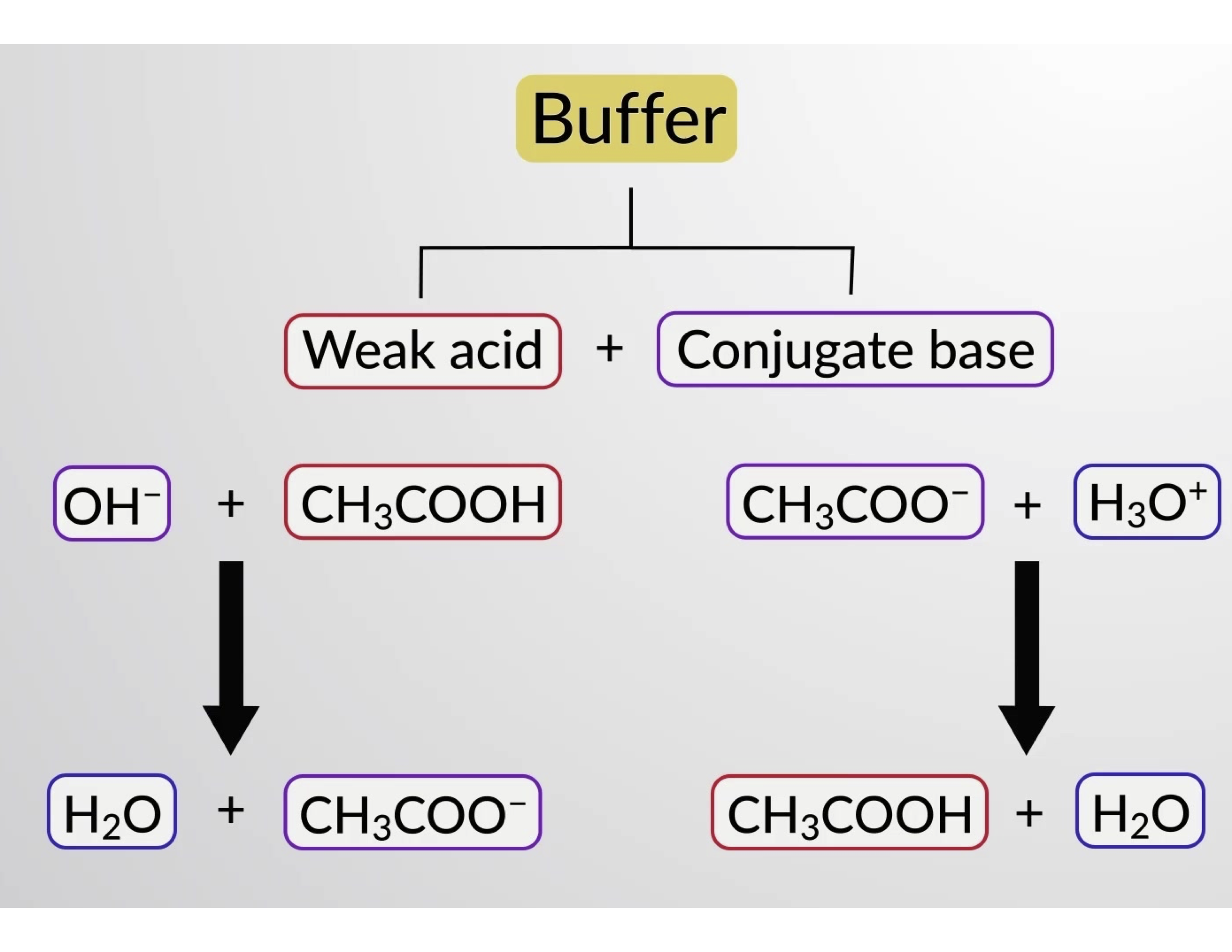
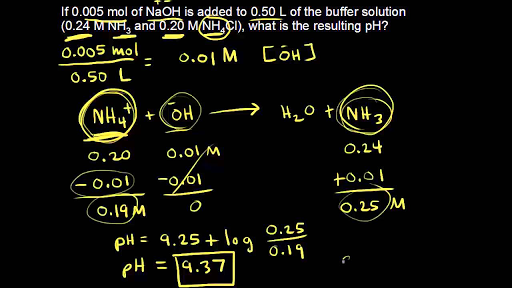
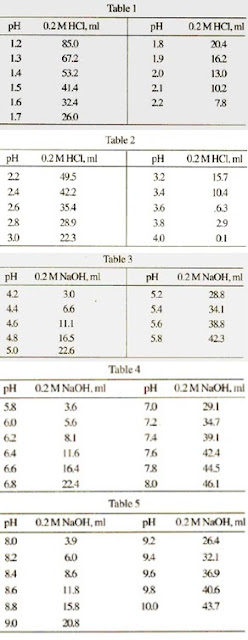
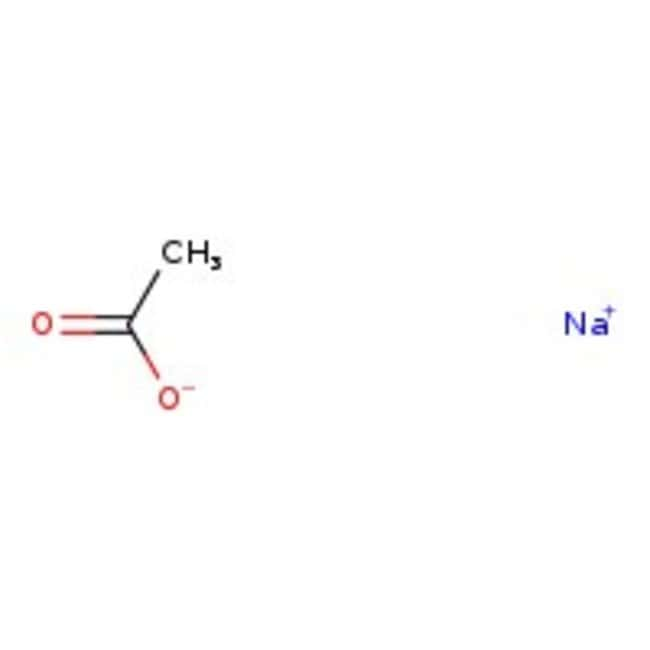
Buffer solution reagent - Acetate 4.0 - GE Healthcare Life Sciences - for molecular biology / liquid / sodium

Buffer solution reagent - Acetate 4.0 - GE Healthcare Life Sciences - for molecular biology / liquid / sodium

SOLUTION TAMPON D'ACÉTATE PH 4,65, ACETATE DE SODIUM / ACIDE ACÉTIQUE, Fluka Honeywell™: Page d'accueil | Fisher Scientific

Spectracer Sodium Acetate Buffer Solution pH 4.5, Fisher Chemical™ Quantity: 1000mL General Purpose Buffers | Fisher Scientific
![Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ] Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]](https://i.ytimg.com/vi/t9B5VgPOTG4/maxresdefault.jpg)